OrthoPure
Introduction
Between my undergraduate years and medical school, I completed a Master’s in the Center for Bioengineering Innovation and Design at Johns Hopkins within the Whiting School of Engineering and the School of Medicine. This intensive one year program is meant to immerse students in the engineering design process within the medical device space from problem identification to solution conceptualization to prototyping/proof of concept, and ultimately, to the bedside and to the market. The year is spent learning about everything from design to patent law to the FDA approval process. As such, there is no better way to learn these skills than to apply them to a real problem. Therefore, as part of the year, I was part of a group of 4 student engineers and an orthopedic surgeon to once again tackle a quite familiar problem to me: the wear of artificial joints, resulting in joint failure and expensive and painful revision surgery.
A Different Perspective
As engineers, when we first approached this problem of wear within an artificial joint, we brainstormed ideas that revolved around tackling the root cause of mechanical failure of the joint. We wanted to essentially design a system to lower the interface force at the joint, and thus, extend its lifetime. However, when we spent dedicated time to brainstorming a broad set of solutions, we came up with something quite different. In discussing the problem, we realized that the failure mechanism of total joint arthroplasty (TJA) begins when submicron wear debris are generated as result of daily use of the implant. These particles undergo endocytosis by macrophage and result in a downstream cascade of signaling that leads to osteoclast activation and bone resorption; leading to loosening of the implant and the need for revision surgery.
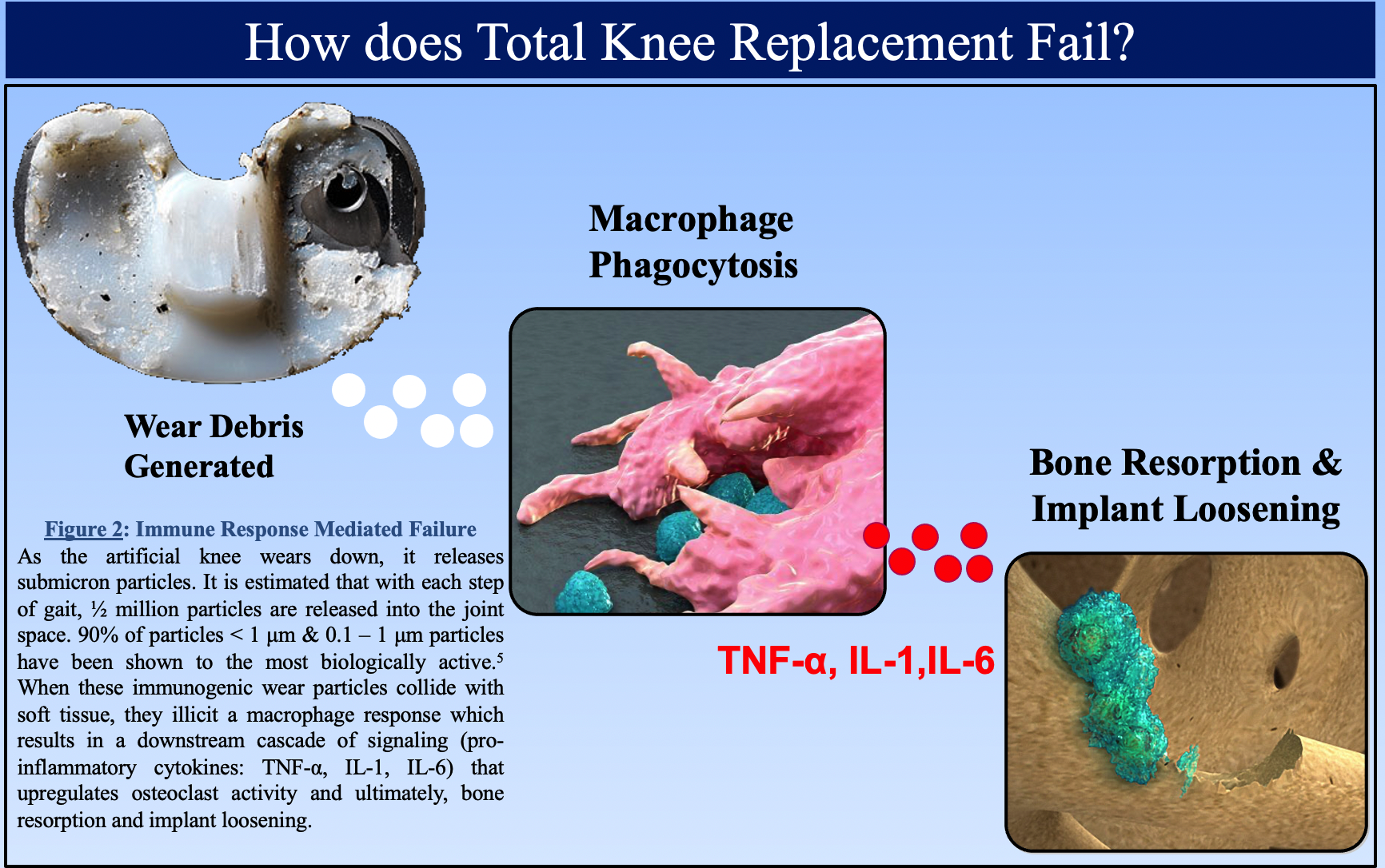
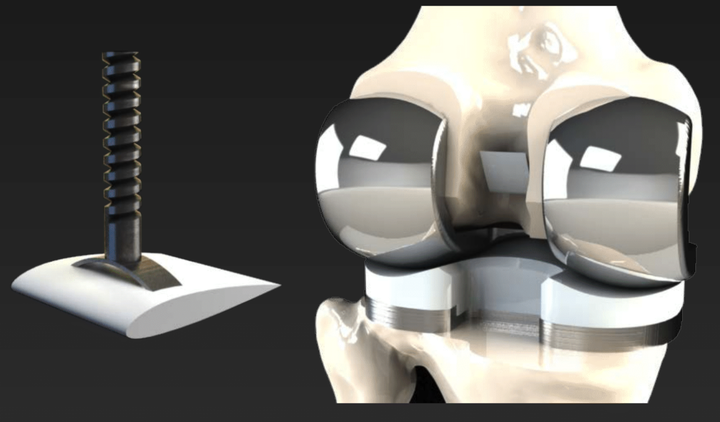
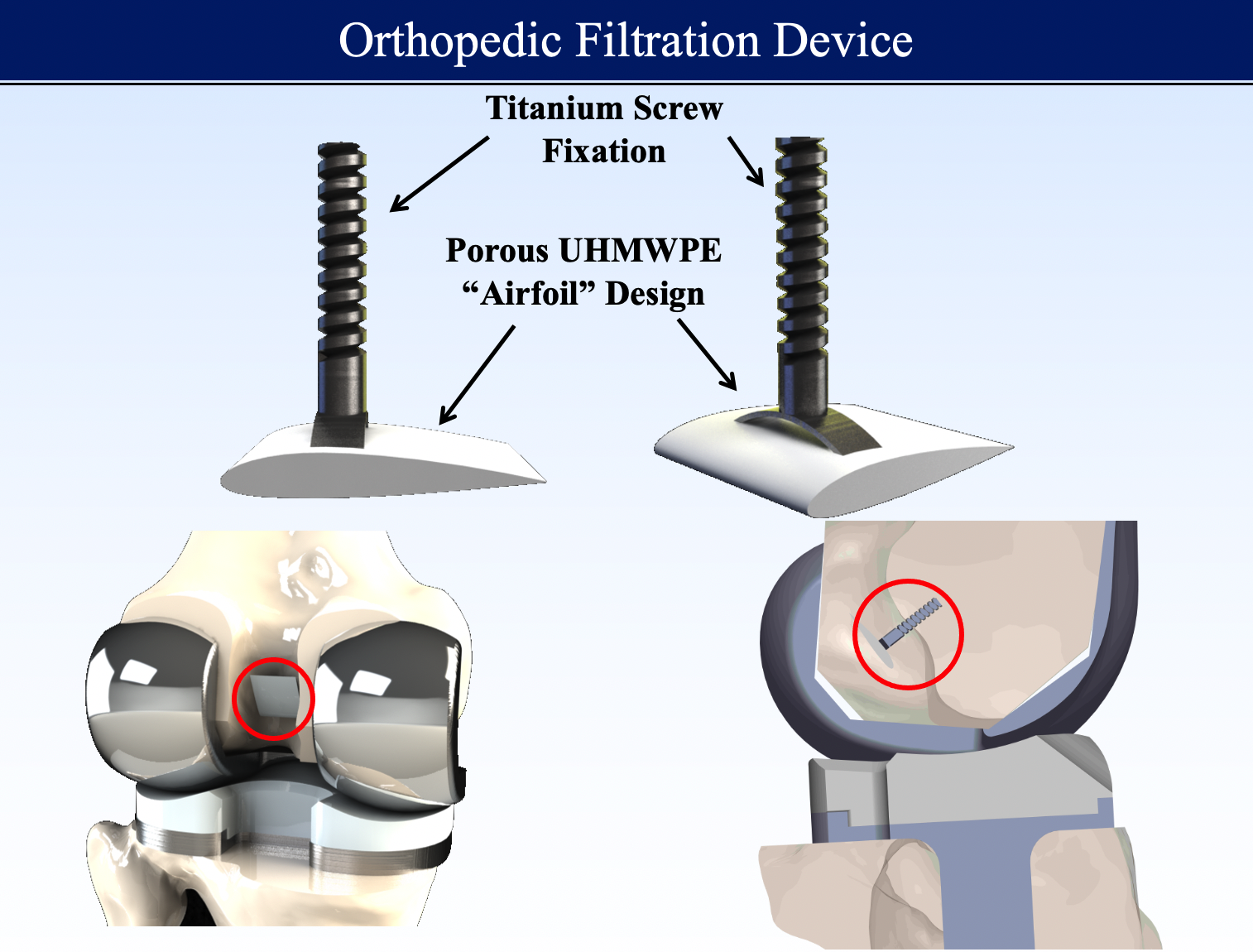
While conventional approaches to this problem have focused around improving materials, we tackled the problem by developing a method of filtering out the immunogenic wear debris to prevent the downstream activation of osteoclasts. The proposed solution of an orthopedic filtration device would consist of two components: 1) porous UHMWPE filtration unit that is currently used and manufactured for other medical filtration applications as well as cranioplasty and 2) a titanium fixation screw for proper placement of the filter. This device would also utilize an “airfoil” design as to take advantage of the Venturi effect to create a pressure gradient in the transverse direction of the filter. This would help optimize the placement and profile of the device to minimize risk of the device causing problems in functionality of the artificial knee implant.
Proof of Concept
In order to see whether a filtration device within the joint space could effectively capture wear debris, we collected bovine serum taken from artificial joint wear simulators (containing wear debris) and setup a device with oscillatory flow to simulate the fluidics of the knee joint.
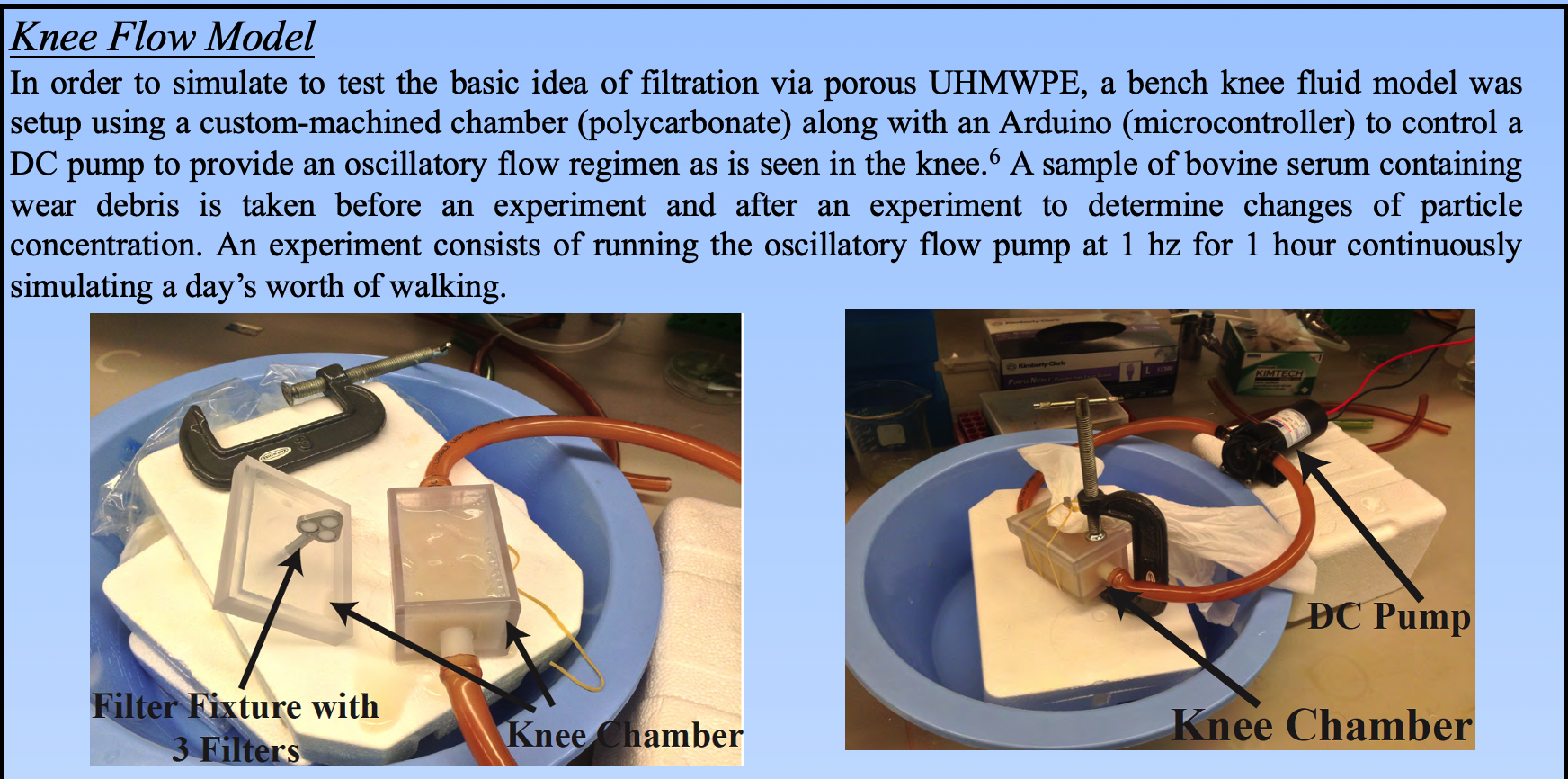
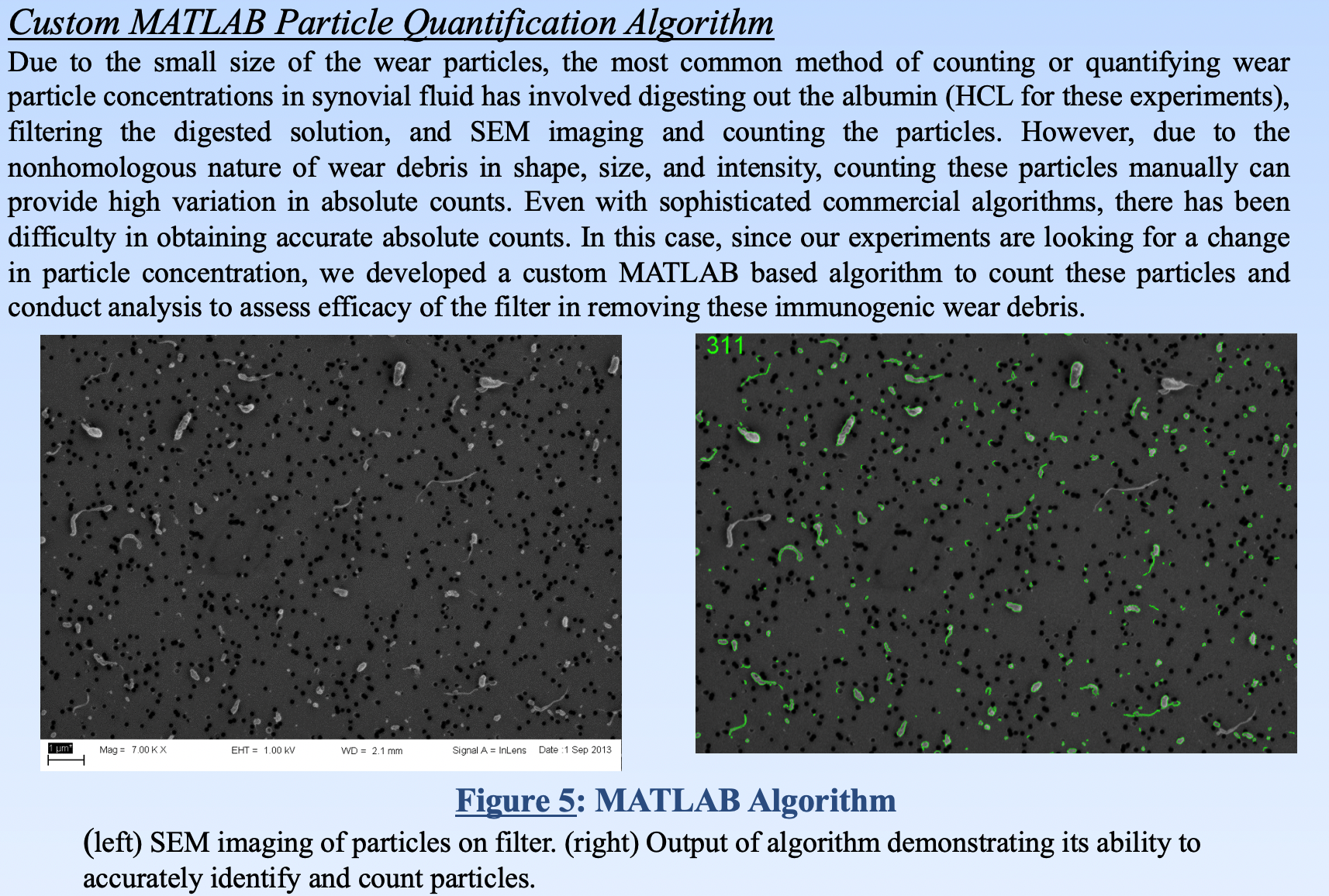
To quantify how much of the debris our device was removing from the synovial fluid, we captured the wear debris on filter paper and used scanning electron microscopy (SEM) to visualize the wear debris. A custom MATLAB algorithm was written to measure the density of wear debris as a surrogate for its concentration within the synovial fluid.
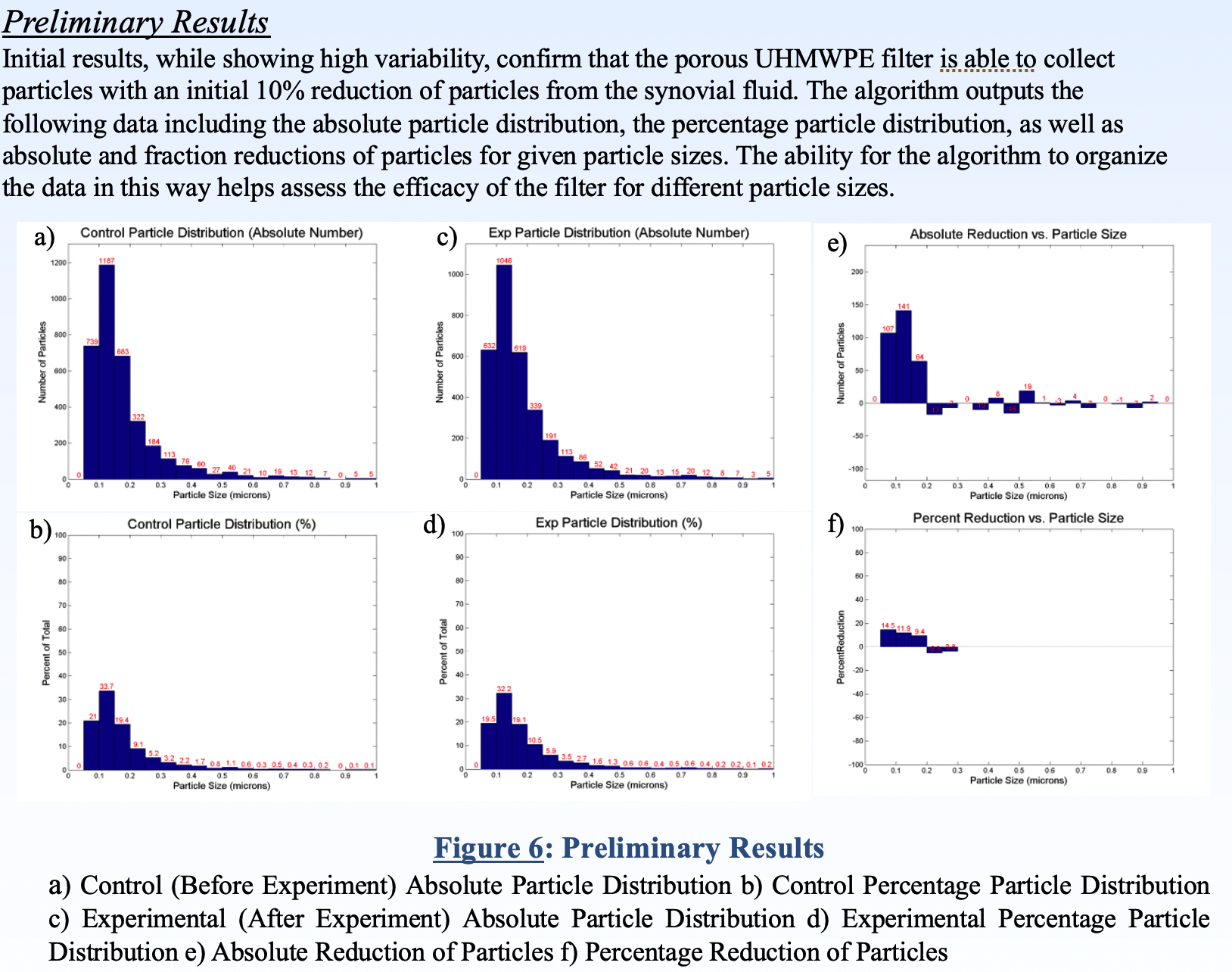
Preliminary results show 10% reduction in wear debris after filtration for 24 hours with one 90µ filter.
These results were presented at the 2013 AMA Conference (poster here).
Reflection
While I did not eventually choose to go into orthopedics as a clinical area, this project sparked my interest in immunology because while wear is perceived as a mechanical failure, the clinical failure of artificial joints is due to the immunologic reaction to the wear debris leading to bone resorption and implant loosening. This realization ultimately pushed me into the field of immunology and eventually, into cancer immunology.